Duration of analgesic relief
PENTHROX® demonstrated lower median pain scores versus standard care at 15, 30, and 60 minute time points.*7
Secondary outcome measures for impact of PENTHROX®
versus standard
care on pain in the ED NRS pain
scores for predefined time points
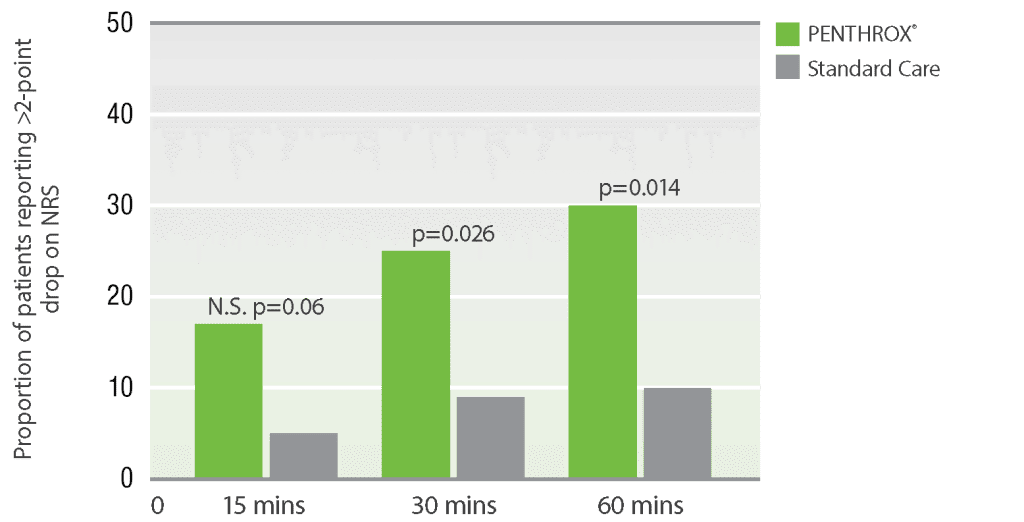
Adapted from Brichko, et al. (2021)
* A randomized, parallel-group, open-label, phase IV trial in 120 adults in a tertiary hospital ED setting. Inclusion criteria required patients to have an initial pain score ≥ 8 on the 11-point NRS. Patients were randomized 1:1 to receive either inhaled PENTHROX® (3 mL) or standard analgesic treatment at ED triage. The primary outcome was the proportion of patients achieving clinically substantial pain reduction, defined as a ≥50% drop in the pain score at 30 minutes. Secondary outcomes included the pain score at multiple time points (15, 30, 60, 90 minutes) and the difference in the proportion of patients achieving a >2-point reduction on the NRS.
ED = Emergency Department
CLINICAL USE:
Due to dose limitations of a treatment course of PENTHROX® and the duration of associated pain relief, PENTHROX® is not appropriate for providing relief of break-through pain in chronic pain conditions. PENTHROX® is also not appropriate for relief of repetitive pain. PENTHROX® is not indicated for use during pregnancy or the peripartum period, including labour.
CONTRAINDICATIONS:
• Altered level of consciousness due to any cause
including head injury, drugs, or alcohol
• Clinically
significant renal impairment
• History of liver
dysfunction after previous methoxyflurane use or
other halogenated anesthetics
• Hypersensitivity to
methoxyflurane or any other halogenated
anesthetics
• Known or genetically susceptible to
malignant hyperthermia or a history of severe adverse
reactions in either patient or relatives
•
Clinically evident hemodynamic instability
•
Clinically evident respiratory impairment
• Use as an
anesthetic agent
MOST SERIOUS WARNINGS AND PRECAUTIONS:
Nephrotoxicity: Supratherapeutic doses of methoxyflurane inhalation have been shown to lead to serious, irreversible nephrotoxicity in a dose-related manner. Dosing limitations should be followed meticulously to prevent or limit risk of nephrotoxicity. Consecutive day use of PENTHROX® is not recommended because of nephrotoxic potential. The lowest effective dose should be administered, especially in the elderly or in patients with other known risk factors of renal disease.
Hepatotoxicity: Very rare cases of hepatotoxicity have been reported with methoxyflurane inhalation when used for analgesic purposes. Use with care in patients with underlying hepatic conditions or having risk factors for hepatic dysfunction. PENTHROX® must not be used in patients who have a history of showing signs of liver damage after previous methoxyflurane use or halogenated hydrocarbon anesthesia.
OTHER RELEVANT WARNINGS AND PRECAUTIONS:
• Potential CNS effects
• Administer with caution in
elderly patients with hypotension and bradycardia due to
possible reduction in blood pressure
• Drug
dependence
• May influence the ability to drive and
operate machinery
• Do not administer concomitantly
with alcohol ingestion
• To reduce occupational
exposure to methoxyflurane, the PENTHROX®
Inhaler should always be used with the activated carbon
chamber to adsorb exhaled methoxyflurane
• Local skin
reactions or irritation to the eyes and mucous
membranes
• Exercise caution if administering to a
nursing mother
FOR MORE INFORMATION:
Please consult the Product Monograph at https://health-products.canada.ca/dpd-bdpp/index-eng.jsp for important information relating to adverse reactions, drug interactions, patient counselling, and dosing/ disposal information (regarding the total maximum dose for a single administration or over the first day of treatment, in a single 48-hour period and entire treatment course) which have not been discussed in this piece.
The Product Monograph is also available by calling us at 1-888-867-7426.
† Comparative clinical significance unknown.
*Clinical significance unknown
REFERENCES:
1.
PENTHROX®Product
Monograph,
Paladin
Pharma
Inc.,
April
2022.
2.
Borobia
AM,
Collado
SG,
Cardona
CC, et
al.
Inhaled
Methoxyflurane
Provides
Greater
Analgesia
and
Faster
Onset of
Action
Versus
Standard
Analgesia
in
Patients
With
Trauma
Pain:
InMEDIATE:
A
Randomized
Controlled
Trial in
Emergency
Departments.
Annals
of
Emergency
Medicine.
2020;75(3):315–28.
3.
Coffey
F,
Wright
J,
Hartshorn
S, et
al.
STOP!: a
randomised,
double-blind,
placebo-controlled
study of
the
efficacy
and
safety
of
methoxyflurane
for the
treatment
of acute
pain.
Emerg
Med J.
2014;31(8):613–8.
4.
Voza A,
Ruggiano
G, Serra
S, et
al.
Inhaled
Methoxyflurane
versus
Intravenous
Morphine
for
Severe
Trauma
Pain in
the
Emergency
Setting:
Subgroup
Analysis
of
MEDITA,
a
Multicenter,
Randomized,
Controlled,
Open-Label
Trial.
JPR.
2020;Volume
13:491–502.
5.
Spruyt
O,
Westerman
D,
Milner
A, et
al. A
randomised,
double-blind,
placebo-controlled
study to
assess
the
safety
and
efficacy
of
methoxyflurane
for
procedural
pain of
a bone
marrow
biopsy.
BMJ
Support
Palliat
Care.
2014;4(4):342–8.
6.
Grummet
J, Huang
S,
Konstantatos
A, et
al. The
‘green
whistle’:
A novel
method
of
analgesia
for
transrectal
prostate
biopsy:
NOVEL
METHOD
OF
ANALGESIA
FOR
TRUS-GUIDED
PROSTATE
BIOPSY.
BJU Int.
2012;110:85–8.
7.
Nguyen
NQ,
Toscano
L,
Lawrence
M, et
al.
Patient-controlled
analgesia
with
inhaled
methoxyflurane
versus
conventional
endoscopist-provided
sedation
for
colonoscopy:
a
randomized
multicenter
trial.
Gastrointestinal
Endoscopy.
2013;78(6):892–901.
8.
Brichko
L,
Gaddam
R, Roman
C, et
al.
Rapid
Administration
of
Methoxyflurane
to
Patients
in the
Emergency
Department
(RAMPED)
Study: A
Randomized
Controlled
Trial of
Methoxyflurane
Versus
Standard
Care.
Miner J,
editor.
Acad
Emerg
Med.
2021;28(2):164–71.